Scientists and business people have been interested in silicon for a long time because it is a very useful element that can be used in many different ways. People have some different ideas about whether it is a metal or non-metal because it has interesting qualities and can be used for many different things.Is silicon metal or nonmetal?
This page delves further into silicon, the semiconductor, discussing its chemical and physical properties, where it is on the periodic table, and how it behaves as a metal and a nonmetal. In order to provide you with an in-depth comprehension of this aspect, we will be analyzing its current and future roles in many disciplines and classifying it accordingly.
Introduction to Silicon
It would be reasonable to assume that ultrapure crystalline silicon is a metal if one were to observe it. Silicone has the appearance of a lustrous metal. However, it is intrinsically brittle at room temperature (a characteristic of non-metals) and a semiconductor, in contrast to metals (more on this below).
The amalgamation of these characteristics categorizes it as a metalloid, an imprecise term denoting an element that is neither metallic nor nonmetallic. Additional elements from this section of the periodic table are also classified as metalloids, including tellurium, boron, germanium, arsenic, antimony, and sometimes a few others, depending on the precise definition of the term.
Definition of Silicon
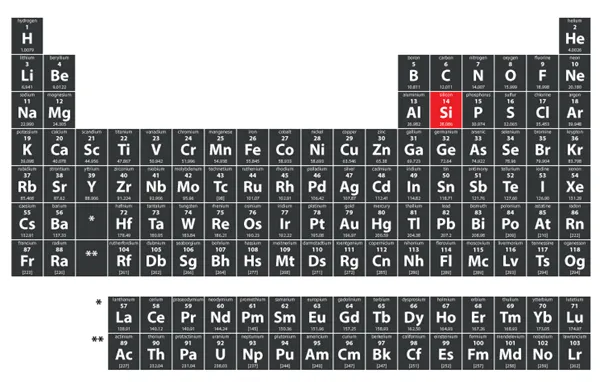
Silicon is a chemical element compound with the symbol Si and an atomic number of 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and it is a member of the carbon family. Also, silicon is classified as a metalloid because some of its properties resemble those of metals and some of its properties resemble those of nonmetals. The important properties of silicon and its classification on the periodic table are discussed in this article, along with some of its key applications.
Properties of Silicon
Understanding the properties of silicon sheds light on its importance across various fields and industries.
- Atomic Symbol: Si
- Atomic Number: 14
- Element Category: Metalloid
- Density: 2.329g/cm3
- Melting Point: 2577°F (1414°C)
- Boiling Point: 5909°F (3265°C)
- Moh’s Hardness: 7
Physical Properties
Silicon is a semi-metal with a melting point of 1414°C and a boiling point of 3265°C. It has a diamond cubic crystal structure, making it a hard and brittle material.
Chemical Properties
Silicon’s ability to form strong covalent bonds makes it a significant component of many modern industrial products. It does not react to most things, although it can react to specific compounds in certain circumstances.
History of Silicon
The Swedish chemist Jons Jacob Berzerlius is credited with first isolating silicon in 1823. Berzerlius accomplished this by heating metallic potassium (which had only been isolated a decade earlier) in a crucible along with potassium fluorosilicate. The result was amorphous silicon.
Making crystalline silicon, however, required more time. An electrolytic sample of crystalline silicon would not be made for another three decades. The first commercialized use of silicon was in the form of ferrosilicon.
Following Henry Bessemer’s modernization of the steelmaking industry in the mid 19th century, there was great interest in steel metallurgy and research in steelmaking techniques. By the time of the first industrial production of ferrosilicon in the 1880s, the importance of silicon in improving ductility in pig iron and deoxidizing steel was fairly well understood.
Early production of ferrosilicon was done in blast furnaces by reducing silicon-containing ores with charcoal, which resulted in silvery pig iron, a ferrosilicon with up to 20 percent silicon content.
The development of electric arc furnaces at the beginning of the 20th century allowed not only greater steel production, but also more ferrosilicon production. In 1903, a group specializing in making the ferroalloy (Compagnie Generate d’Electrochimie) began operations in Germany, France and Austria and, in 1907, the first commercial silicon plant in the US was founded.
Steelmaking was not the only application for silicon compounds commercialized before the end of the 19th century. To produce artificial diamonds in 1890, Edward Goodrich Acheson heated aluminum silicate with powdered coke and incidentally produced silicon carbide (SiC).
Three years later Acheson had patented his production method and founded Carborundum Company (carborundum being the common name for silicon carbide at the time) for the purpose of making and selling abrasive products.
By the early 20th century, silicon carbide’s conductive properties had also been realized, and the compound was used as a detector in early ship radios. A patent for silicon crystal detectors was granted to GW Pickard in 1906.
In 1907, the first light emitting diode (LED) was created by applying voltage to a silicon carbide crystal. Through the 1930s silicon use grew with the development of new chemical products, including silanes and silicones. The growth of electronics over the past century has also been inextricably linked to silicon and its unique properties.
While the creation of the first transistors – the precursors to modern microchips – in the 1940s relied on germanium, it was not long before silicon supplanted its metalloid cousin as a more durable substrate semiconductor material. Bell Labs and Texas Instruments began commercially producing silicon-based transistors in 1954.
The first silicon integrated circuits were made in the 1960s and, by the 1970s, silicon-containing processors had been developed. Given that silicon-based semiconductor technology forms the backbone of modern electronics and computing, it should be no surprise that we refer to the hub of activity for this industry as ‘Silicon Valley.’
(For a detailed look at the history and development of Silicon Valley and microchip technology, I highly recommend the American Experience documentary entitled Silicon Valley). Not long after unveiling the first transistors, Bell Labs’ work with silicon led to a second major breakthrough in 1954: The first silicon photovoltaic (solar) cell.
Prior to this, the thought of harnessing energy from the sun to create power on earth was believed impossible by most. But just four years later, in 1958, the first satellite powered by silicon solar cells was orbiting the earth.
By the 1970s, commercial applications for solar technologies had grown to terrestrial applications such as powering lighting on offshore oil-rigs and railroad crossings. Over the past two decades, the use of solar energy has grown exponentially. Today, silicon-based photovoltaic technologies account for about 90 percent of the global solar energy market.
Classification of Silicon
Discover where silicon sits in the periodic table and how it compares to other elements.
Silicon is located in group 14 of the periodic table, known as the carbon group. It shares similarities with carbon due to its ability to form multiple stable covalent compounds.
Comparison with the Other Elements
Silicon does not conduct electricity like metals do, but it does have some metallic properties that make it hard to tell the difference between metal and non-metal qualities.
Metallic Characteristics
While silicon is not a metal in the traditional sense, it exhibits some metallic properties, such as brittleness and a metallic luster. These properties make it essential in the manufacturing of various electronic devices.
Conductivity and Structural Properties
Silicon is a semiconductor with a unique ability to conduct electricity under specific conditions. Its crystalline structure plays a vital role in its applications in electronics, solar cells, and other technological advancements.
Silicon as a Non-Metal
Non-Metallic Characteristics
Silicon, despite its metalloid status, leans more towards being a non-metal due to its properties. It lacks the typical luster and conductivity of metals. Instead, silicon is brittle in its solid form and behaves more like a non-metal in chemical reactions.
Bonding and Reactivity
Silicon bonds covalently like non-metals, sharing electrons to form strong molecular structures. Its reactivity is more akin to non-metals, forming compounds with elements like oxygen rather than metallic alloys.
Applications of Silicon in Industry
Electronics and Semiconductor Industry
Silicon’s primary claim to fame is in the electronics industry where it serves as the foundation for semiconductors. Its unique semiconducting properties make it essential for integrated circuits and microchips.
Solar Energy and Photovoltaics
Silicon plays a crucial role in solar energy technology, particularly in photovoltaic cells. These cells convert sunlight into electricity, making silicon a key player in the renewable energy sector.
Future Trends and Developments in Silicon
Advancements in Silicon Technology
As technology advances, so does silicon. Researchers are constantly exploring ways to improve silicon’s efficiency and performance in various applications, driving innovations in electronics, solar energy, and beyond.
Potential Innovations and Research Areas
Future developments may include enhanced silicon-based materials, novel manufacturing techniques, and exploring silicon’s potential in emerging technologies like quantum computing and biotechnology.
Conclusion
In the grand debate of whether silicon is a metal or non-metal, its inclination towards non-metallic behavior and widespread industrial applications solidifies its importance in modern technology. As research continues to push the boundaries of silicon’s capabilities, its role in shaping the future of technology remains indispensable. In conclusion, the classification of silicon as a metal or non-metal remains a topic of interest and debate within the scientific community. Its unique properties and widespread applications in industries such as electronics, solar energy, and beyond continue to drive research and innovation. As advancements in silicon technology progress, the future holds promising developments that will further enhance our understanding and utilization of this remarkable element.
Frequently Asked Questions (FAQ)
1. Is silicon considered a metal or a non-metal?
Silicon is typically classified as a metalloid, exhibiting properties of both metals and non-metals.
2. How is silicon used in the electronics industry?
Silicon is a key component in semiconductor devices, such as transistors and integrated circuits, which form the building blocks of modern electronic devices.
3. What are some potential future applications of silicon technology?
Advancements in silicon technology may lead to innovations in areas such as renewable energy, bioengineering, and quantum computing, among others.